Facts without context
Those opposed to animal research often point out that most drugs that pass the legally required toxicology tests in animals go on to fail in human clinical trials. They then go on to suggest that this shows that animal research does not work, or that it is proof that animals are not accurate models for humans.
However, this is misleading without an understanding of the relevant context and the reasons for the animal safety tests. Ironically, the figures cited by many animal rights activists are actually drawn from industry and are intended to explain the expense of developing safe and useful medicines.
The most frequently used statistics are
- “90% drugs tested on animals fail” – British Union for the Abolition of Vivisection
- “92% of drugs fail in clinical trials, having successfully passed through animal studies” – Safer Medicines Trust
- “In fact according to the FDA’s research, nine out of ten drugs deemed successful in animal tests fail in human clinical trials” – Humane Society International
The main sources of this information come from the US Food and Drug Administration (FDA). In 2006, health and Human Services Secretary, Mike Leavitt said:
“Currently, nine out of ten experimental drugs fail in clinical studies because we cannot accurately predict how they will behave in people based on laboratory and animal studies,”
The 92% statistic comes from an earlier report which showed only 8% of those drugs passing animal testing stages would go on successfully to be FDA approved.
“For example, a new medicinal compound entering Phase 1 testing, often representing the culmination of upwards of a decade of preclinical screening and evaluation, is estimated to have only an 8 percent chance of reaching the market.” – Challenges and Opportunities Report, FDA, 2004
Are the figures right?
To help break down these statistics, it is useful to look at the success rates at each stage. In the diagram below the red percentages show the proportion of drugs that move from one stage to another – so 64% of New Molecular Entities (NMEs – essentially new drugs) will pass the animal tests (preclinical studies) and be moved into Phase 1 clinical trials in humans.
Looked at this another way, animal experiments remove 36% of the potential drugs from moving onto the next stage. This is almost certainly a good thing as it avoids humans being given drugs which are likely to be toxic to them. The percentages at the bottom look at the percentage chance that a drug that has made it to that stage will make it all the way – so of all the drugs that make it to Phase 2 clinical trials, 12% will be approved by the FDA (and 88% will fail).
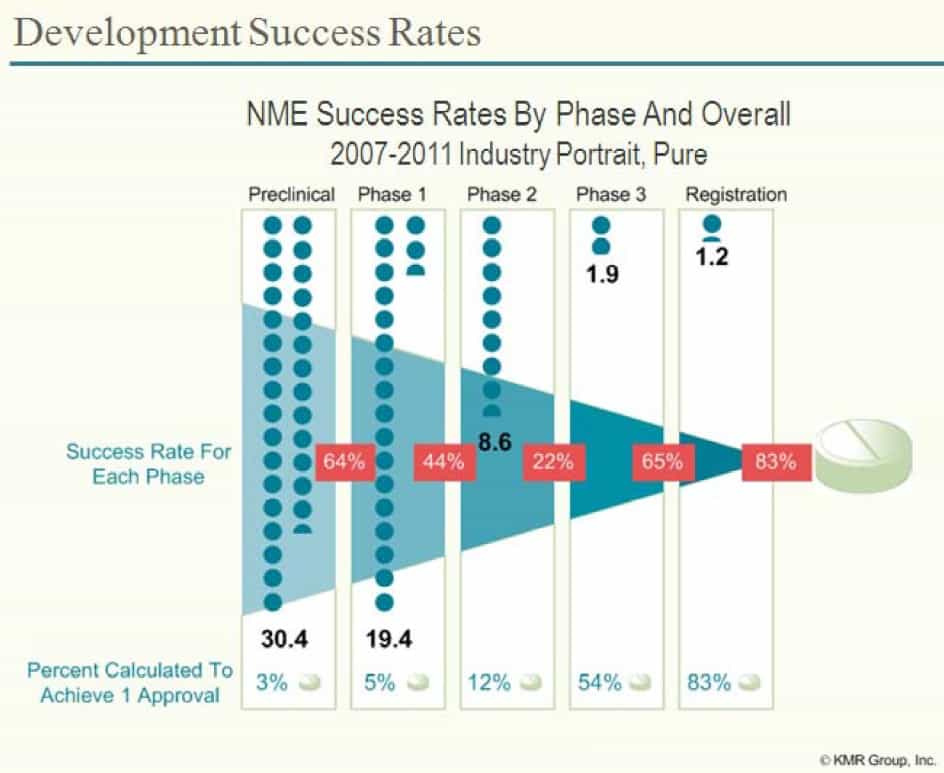
The first thing to note is of those drugs which pass animal tests, 94% will fail during human clinical trials stages (Phases 1 – 3)*.
* number of drugs which have passed Phase 3 clinical trials ÷ number of drugs which have passed just animal tests = 1.2 ÷ 19.4 = 6.2% of drugs which reach Phase 1 trials are eliminated by Phase 1-3 clinical trials. 100 – 6.2 = 93.8% fail.
So the failure rate is actually higher than even the animal rights organisations suggest (since they are using data from before 2006). Is this damning for animal research?
Consider that of all the drugs which pass Phase 1 clinical trials in humans, 86% will fail in later stage human trials**. Yet, we do not hear activists suggesting that humans are an entirely inappropriate model for drug development (though we should note that one human is not a perfect model for another).
** number of drugs which have passed Phase 3 clinical trials ÷ number of drugs which have passed Phase 1 trials = 1.2 ÷ / 8.6 = 14% of drugs which pass Phase 1 trials are eliminated by Phase 2-3 clinical trials. 100 – 14 = 86% fail.
Facts with context
Here is where it is important to understand a little about the drug development process.Before the preclinical animal tests there are a large number of pre-preclinical non-animal tests done on all manner of research tools including computer models, automatic screening, cell cultures, microbial studies and more. These methods are used to (relatively) cheaply remove many potentially toxic, or obviously non-starting drugs from reaching the more expensive animal testing stage – greatly reducing the amount of animal research required for a drug to reach market.
So contrary to animal rights claims of alternative methods being better, the truth is that 94% of drugs that pass animal AND non-animal preclinical tests will fail in human tests.So rather than damn just the animal tests, have animal rights activists managed to damn all of preclinical research? In short, no.
The role of preclinical animal tests is to check if the drug offers any potential therapeutic value and, importantly, if it is safe enough to move to Phase 1 trials in humans. This does not even mean free of all side effects, but to learn whether a drug can safely be given to humans and at what approximate dosage.
If you want to know how truly successful animal tests are, consider that in over 30 years there has not been a single death in a Phase 1 clinical trial in the UK. The last major incident was in 2006 in the Northwick Park trials where 6 people suffered extreme side effects in a Phase 1 clinical trial – though it should be noted that TGN1412 was a very novel type of molecule which was poorly understood. Considering that there are normally over 200 Phase I clinical trials each year in the UK (each involving multiple people), animal testing has been exceptionally effective at keeping dangerous drugs away from people.
Even Phase 1 clinical trials in humans are not intended to check for efficacy, but rather to assess whether a drug is safe enough to be tried in a larger number of patients (who are suffering from an illness the drug is intended to treat).
Furthermore, when a drug is licensed for use, it is on the basis of the clinical trials in humans, not the preclinical animal tests which exist to ensure that a drug is safe enough to move into Phase 1 trials. So when animal rights activists claim that adverse drug reactions can be blamed on animal tests approving the drug, remember that it is the clinical trials in thousands of people which provide the evidence of its safety.
Professor Robin Lovell-Badge is Head of the Division of Stem Cell Biology and Developmental Genetics, MRC National Institute for Medical Research, London.
Last edited: 6 April 2022 13:02