-
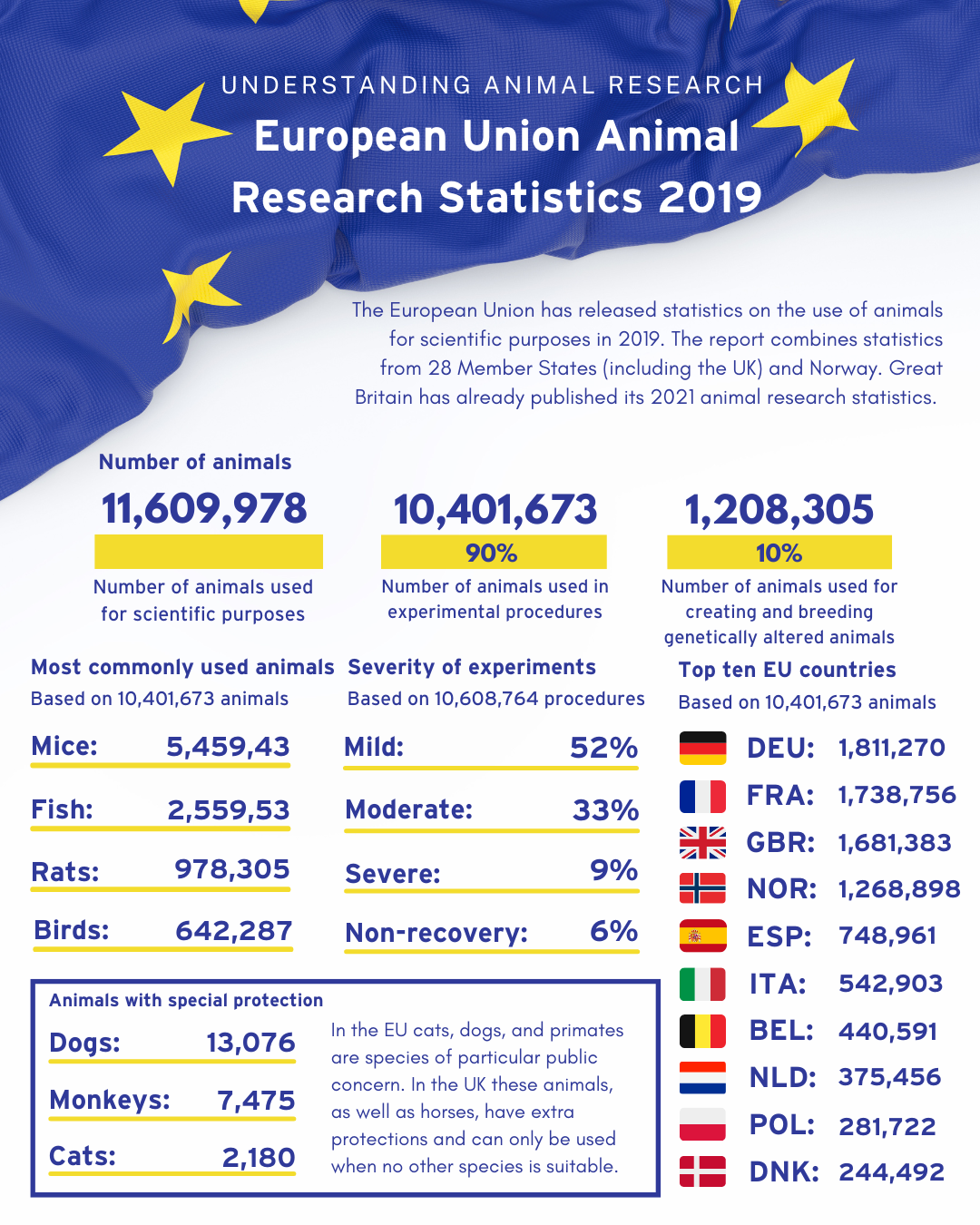
Number of animals used for research in the EU (and Norway) in 2019 has decreased by 4% to 11,609,978
-
Mice, fish, rats and birds account for 93% of animals used
-
Cats, dogs, and primates account for 0.22%
-
Germany was the highest user of animals
-
Great Britain has already published its 2021 animal research statistics
Today (Tuesday, 19 July 2022) the European Commission has released its ‘report on the statistics on the use of animals for scientific purposes in the Member States of the European Union and Norway in 2019’. The figures show that 11,609,978 animals were used for scientific, medical and veterinary research across 28 Member States (including the UK) and Norway in 2019, 4% less than in 2018.
The three countries that used the highest number of animals for experimental purposes in 2019 were:
- Germany: 1,811,270 animals used (17.4%)
- France: 1,738,756 animals used (16.7%)
- UK: 1,681,383 animals used (16.2%)
Click here for more information on country-specific statistics.
Purpose of animals used
Of the 11,609,978 animals used in research in 2019, 10,40,1673 animals (90%) were used for experimental purposes while 1,208,305 animals (10%) were used for the creation and breeding of genetically altered animals. Animals used for experimental purposes decreased by 1.6% compared to 2018, while animals used for the creation and breeding of genetically altered animals decreased by 20.5%.
93% of animals used for experimental purposes in 2019 were mice, fish, rats, and birds, whereas cats, dogs, and primates accounted for 0.22%.
Number of Animal Used for Experimental Purposes in the EU and Norway in 2019
| Species | Number of animals used for experimental purposes (2019) | % of total | % change from 2018 |
| Mice | 5,459,433 | 52.5% | -0.8% |
| Fish | 2,559,532 | 24.6% | -7.5% |
| Rats | 978,305 | 9.4% | -2.1% |
| Birds | 642,287 | 6.2% | 10.2% |
| Other mammals | 673,629 | 6.5% | 2.4% |
| Reptiles | 2,012 | 0.02% | 22.1% |
| Amphibians | 46,776 | 0.5% | 69.5% |
| Primates | 7,475 | 0.07% | -12.9% |
| Cats | 2,180 | 0.02% | 40.3% |
| Dogs | 13,076 | 0.1% | -26.2% |
| Cephalopods | 16,968 | 0.2% | 297.6% |
| TOTAL | 10,401,673 | -1.6% |
Experimental purposes: using animals in scientific studies for purposes such as basic research and the development of treatments, safety testing of pharmaceuticals and other substances, education, specific surgical training and education, environmental research and species protection.
Some animals are used more than once during research and in 2019 there were 10,608,764 procedures carried out on animals for experimental purposes, a 1.8% decrease compared to 2018. This includes basic research, which expands our knowledge of living organisms and the environment; applied research, which addresses the prevention of disease and development of treatments; and regulatory research, which includes studies aimed at ensuring product safety and the effectiveness of pharmaceuticals. Of these 10,608,764 procedures:
-
45% were for basic research
-
27% were for applied research
-
17% were for regulatory research
Creation and breeding of genetically altered animals: the breeding of animals whose genes have mutated or have been modified. These animals are used to produce genetically altered offspring for use in experimental procedures but are not themselves used in experimental procedures.
10% (1,208,305) of all animals used for research in 2019 were for the creation and breeding of genetically altered animals. Of these 1,329,263 animals used:
-
58% were for the purpose of maintenance of established lines of genetically altered animals
-
42% were for the creation of new lines of genetically altered animals
Severity of procedures for experimental purposes
Severity assessments measure the harm experienced by an animal during a procedure. A procedure can be as mild as an injection, or as severe as an organ transplant. More than half (52%) of experimental procedures were classed as (up to and including) mild in 2019.
Severity of Animal Procedures for Experimental Purposes in the EU and Norway in 2019
|
Severity |
Number of procedures for experimental purposes (2019) |
% of total |
Change from 2018 |
|
Non-recovery |
586,373 |
6% |
= |
|
Mild (up to and including) |
5,512,134 |
52% |
↑ |
|
Moderate |
3,510,993 |
33% |
↓ |
|
Severe |
999,264 |
9% |
↓ |
|
TOTAL |
10,608,764 |
|
↓ |
Severity assessments reflect the peak severity of the entire procedure and are classified into four different categories:
Non-recovery: When the entire procedure takes place under general anaesthetic and the animal is humanely killed before waking up.
Mild (up to and including): The procedure caused short-term mild pain, suffering or distress; or no significant impairment of the well-being or general condition of the animal. For example, the equivalent of an injection or having a blood sample taken. This category also includes any animals which have not experienced a level of pain, suffering, distress or lasting harm above the minimum threshold i.e. the equivalent caused by the introduction of a needle in accordance with good veterinary practice.
Moderate: The procedure caused short-term moderate pain, suffering or distress; long-lasting mild pain, suffering or distress; or moderate impairment of the well-being or general condition of the animal. For example, surgery carried out under general anaesthesia followed by painkillers during recovery.
Severe: The procedure caused severe pain, suffering or distress; long-lasting moderate pain, suffering or distress; or severe impairment of the well-being or general condition of the animal. This would usually include long-term disease processes where assistance with normal activities such as feeding and drinking were required, or where significant deficits in behaviours/activities persist. Animals found dead are commonly classified as severe as pre-mortality suffering often cannot be assessed. Most severe procedures arise in regulatory testing such as evaluation of toxicity of drugs.
UK statistics
Since supplying their figures to the EU, some countries, including the UK, have also published statistics for 2020 and 2021. Statistics on animal use in 2021 for Great Britain can be found here. The EU will continue to publish these EU statistics annually. However, as a result of the UK leaving the EU, the data from the UK will no longer be included from 2020 onwards.
In the EU animal research is strictly regulated under the EU Directive 2010/63. Every procedure, from a simple blood test to major surgery, requires individual, establishment and project licences, as well as approval from animal welfare and ethical review bodies.
All organisations are committed to the ‘3Rs’ of replacement, reduction and refinement. This means avoiding or replacing the use of animals where possible; minimising the number of animals used per experiment and optimising the experience of the animals to improve animal welfare.
The use of animals to test tobacco products was banned in the UK in 1997 and it has been illegal to use animals to test cosmetic products in this country since 1998. A policy ban on household product testing using animals was introduced in 2010. Since 2013, it has been illegal to sell or import cosmetics anywhere in the EU where the finished product or its ingredients have been tested on animals.
Last edited: 27 October 2022 18:28




