As the human population grows older, the challenge presented by disorders of the ageing body and brain, such as Parkinson’s disease, becomes ever greater. In 2020, an estimated 145,000 people lived with Parkinson's in the UK. But in the years to come those numbers are expected to grow and reach 172,000 by 2030. It is essential we understand more about this disease of the brain to find better ways to prevent and even cure it.
Researchers have now spent decades trying to alleviate suffering and distress caused by Parkinson’s – and have in some ways succeeded. But there is still a long road ahead.
Understanding a complex disease
Parkinson’s disease is a disorder of the brain that leads to difficulties in movement. It causes tremors, shaking, stiffness, and difficulties with walking, balance and coordination that usually begin gradually and get worse over time. This is often accompanied by non-movement problems such as poor sleep quality, intestinal dysfunction and even a poor sense of smell, as well as changes in the way sufferers react to reward or motivation and sometimes a deterioration in their ability to understand complex ideas and to make plans.
We now know that the root cause of these symptoms is the death of the brain cells that produce the dopamine neurotransmitter. As we age, we naturally lose a few of these dopamine-producing cells, but this loss somehow accelerates in patients with Parkinson’s and far exceeds what is normally expected.
Arvid Carlsson discovered dopamine in the 1950s using a variety of animal models. He showed that dopamine was involved in the nerve signals responsible for movement and that dopamine dysfunction could result in serious disorders such as Parkinson’s disease. He was awarded the 2000 Nobel Prize for Physiology or Medicine together with Paul Greengard who used animal models to investigate the mechanism of action of dopamine and other neurotransmitters, and Eric Kandel who used sea slugs to study the role of synaptic transmission in learning and memory.
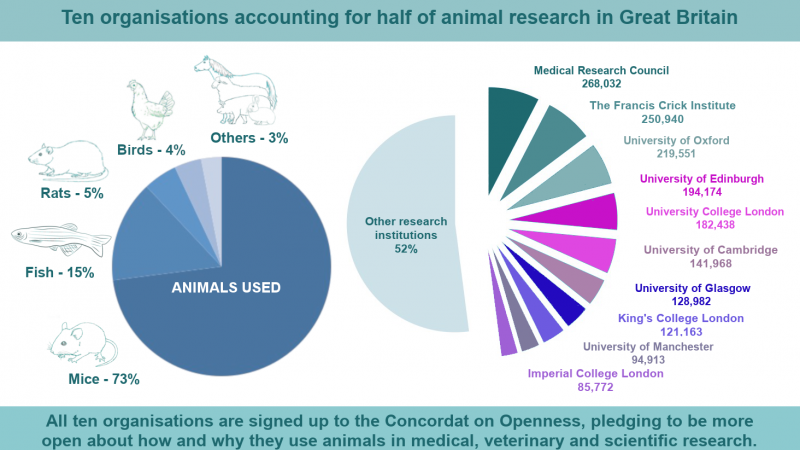
Much of what we know about Parkinson's disease comes from models of the disease in animals, usually mice.
Finding a treatment for Parkinson’s
Once the link between Parkinson’s disease and a lack of dopamine in the brain was made clear, scientists and clinicians worked on replacing the missing neurotransmitter. They found the first effective treatment for Parkinson's in a molecule often called L-dopa. This works brilliantly for some symptoms, but its benefits wear off over time.
“As a field, we're thinking about what new symptomatic treatment could help when the L-dopa no longer works properly. But also we want to get to the root cause, so we can stop the disease all together,” explains Peter Magill, Professor of Neurobiology and Deputy Director of the MRC Brain Network Dynamics Unit at the University of Oxford.
Prof Magill and his team are exploring one of the most dramatic approaches to minimising drug dosages and side effects while maximising benefits, a surgical technique called deep brain stimulation.
“The brain is a big organ. If you want to stimulate it with a metal rod, you have to do it in the right place, the right way and for the right reasons. Animal research was critical for answering those questions. It provided the foundational knowledge and some of the first proofs of principle for stimulating the brain for therapy in Parkinson's,” explains Prof Magill.
Work in animals, in parallel with work in humans, was essential to developing our understanding of how parts of the brain are interconnected and the role of dopamine in those connections. Researchers were able to map out how nerve cells in the wider basal ganglia – the part of the brain that is affected in Parkinson’s disease – are connected and can interact. This helped them identify the part of the network that was affected and needed to be targeted during surgery.
What are we still looking for?
The drugs and surgical interventions that are available for Parkinson’s are effective for many patients but they aren’t perfect. Parkinson’s can have a devastating effect on the quality of life and so it is pressingly important to find new therapies that counteract the symptoms of the illness, even if we can’t – yet – cure it.
However, prevention is better than a cure. Parkinson’s symptoms usually appear when nearly 70% of a patient’s neurons have already died. If we could intervene earlier, outcomes could be much better. Researchers working on finding predictive indicators of the disease hope to be able to develop early interventions to limit the progression of symptoms. The idea is to stop decay before it goes too far, rather than reverse cell death that has already happened.
“I am confident that, as our understanding of the disease progresses, we will find improved symptomatic therapies, more sophisticated ways to interact with the brain using devices, and more precise ways of targeting specific chemical processes in the brain with drugs. It is a really difficult challenge and requires scientists and clinicians to tackle it from many angles. You can look at nerve cells in a dish, or study the human brain, but really the work in animals is fundamental in many ways,” explains Prof Magill.
Animal models of Parkinson’s disease
Parkinson’s researchers are well aware of the limits of animal models, but currently have no research method that can improve on them.
“I don't know of an animal species that naturally develops anything like Parkinson's,” explains Prof Magill. “Parkinson’s disease is very complex, and it is difficult to capture those intricacies in a single animal model. As such, researchers have different ways to model the disease in animals.”
One model of the disease uses a toxin injected into the basal ganglia that kills dopamine neurons in the brain. It mimics the later stages of the disease when there is already a massive loss of dopamine neurons, but doesn’t quite encapsulate the cognitive and genetic features of the illness, nor does it model the symptoms that can be experienced in the gut.
Animal models of Parkinson’s disease cannot be expected to faithfully replicate or recapitulate all the features of a very complex human disease. As such, various animal models answer different questions relating to the disease. One other way to study the disease is to create genetically engineered animals using genes that are thought to predispose humans to the disease.
“For some questions, fruit flies are great, for others tiny worms called C. elegans might be. When looking at very large and complex networks of nerve cells, you might want to turn to mammals, such as rats and mice. We know a lot about mouse genetics and how to change their DNA which makes them a really popular mammalian model. 20 or 30 years ago, it was quite common to use cats in neuroscience, including to model Parkinson’s, and sometimes dogs and non-human primates. That is, of course, no longer the case. Although macaque monkeys are still used in small numbers around the world, their participation in research is heavily governed. Mice were and still are, by far, the most regularly used animal model in neuroscience in general.”
There is still a lot of money and scientific effort being invested in Parkinson’s research, and the use of animal models remains an essential part of the struggle to find new treatments that could transform patient care in years to come.
“Of course, ethically and by law, we try to ensure that we only use animals when we really have to, when they are the only way we can get answers to the scientific questions we have. And for now, I don't know of a way we could replace animals and still get the the answers to the questions we need to answer. In my research lifetime, in the next 20 or 30 years, I do not envisage that we will be able to drop animal research completely. They're too vital in the effort.”
Last edited: 26 April 2022 11:19